- PRODUCTS
- Ultra High Molecular Weight Polyethylene (UHMWPE)
- UHMWPE Products with Vitamin E
- Acetal (Hostaform® MT®2U06)
- Radel® R-5500
- Crosslinking & Crosslinked Polyethylene
- Direct Compression Moulding (DCM)
- Component Machining Products
Introduction
Industry leading research and development
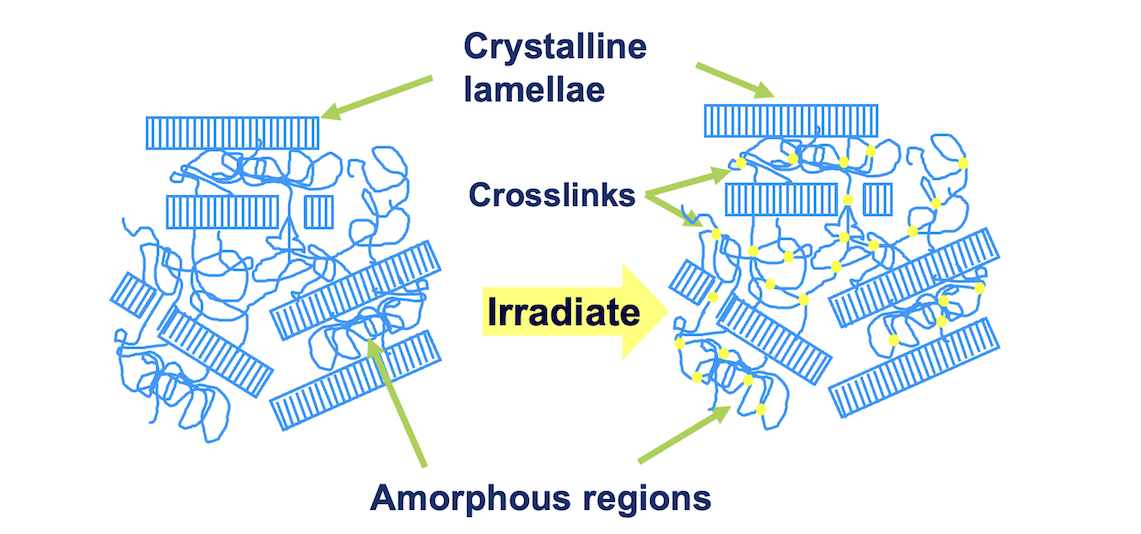
A direct consequence of industry leading research and development activities has allowed Orthoplastics to successfully ensure that they have the capability to manufacture crosslinked UHMWPE.
The benefit of manufacturing UHMWPE in this manner, is a significant reduction in wear on the medical application. By using a process which exposes standard UHMWPE to ionising radiation, it allows Orthoplastics to produce a higher grade of UHMWPE, otherwise referred to as Crosslinked UHMWPE, bringing major benefits to medical implant manufacturers, and the patient community.